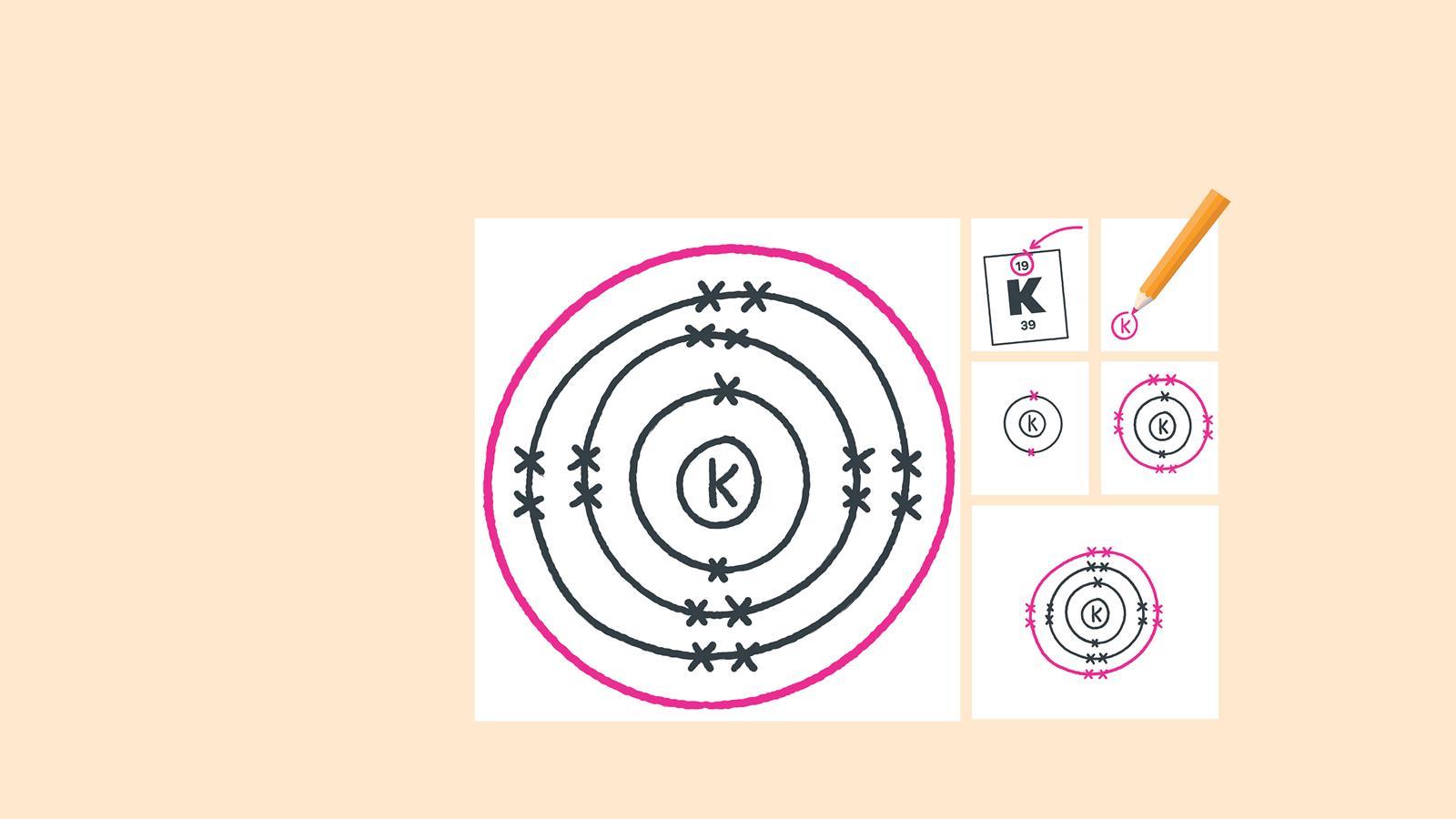
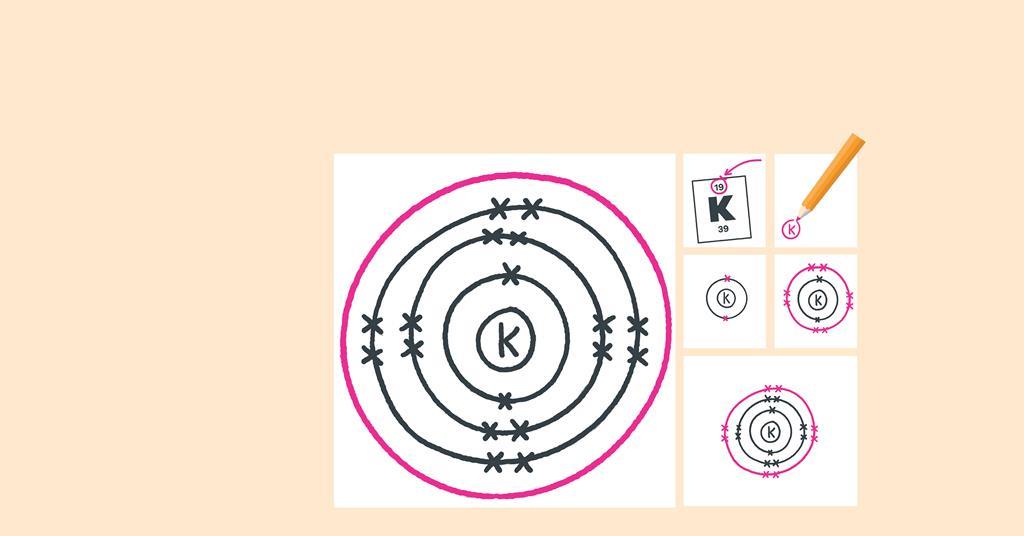
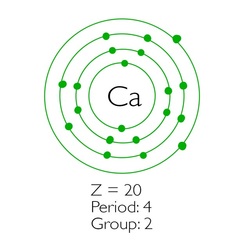
Out Of This World Tips About How To Draw An Electron Configuration
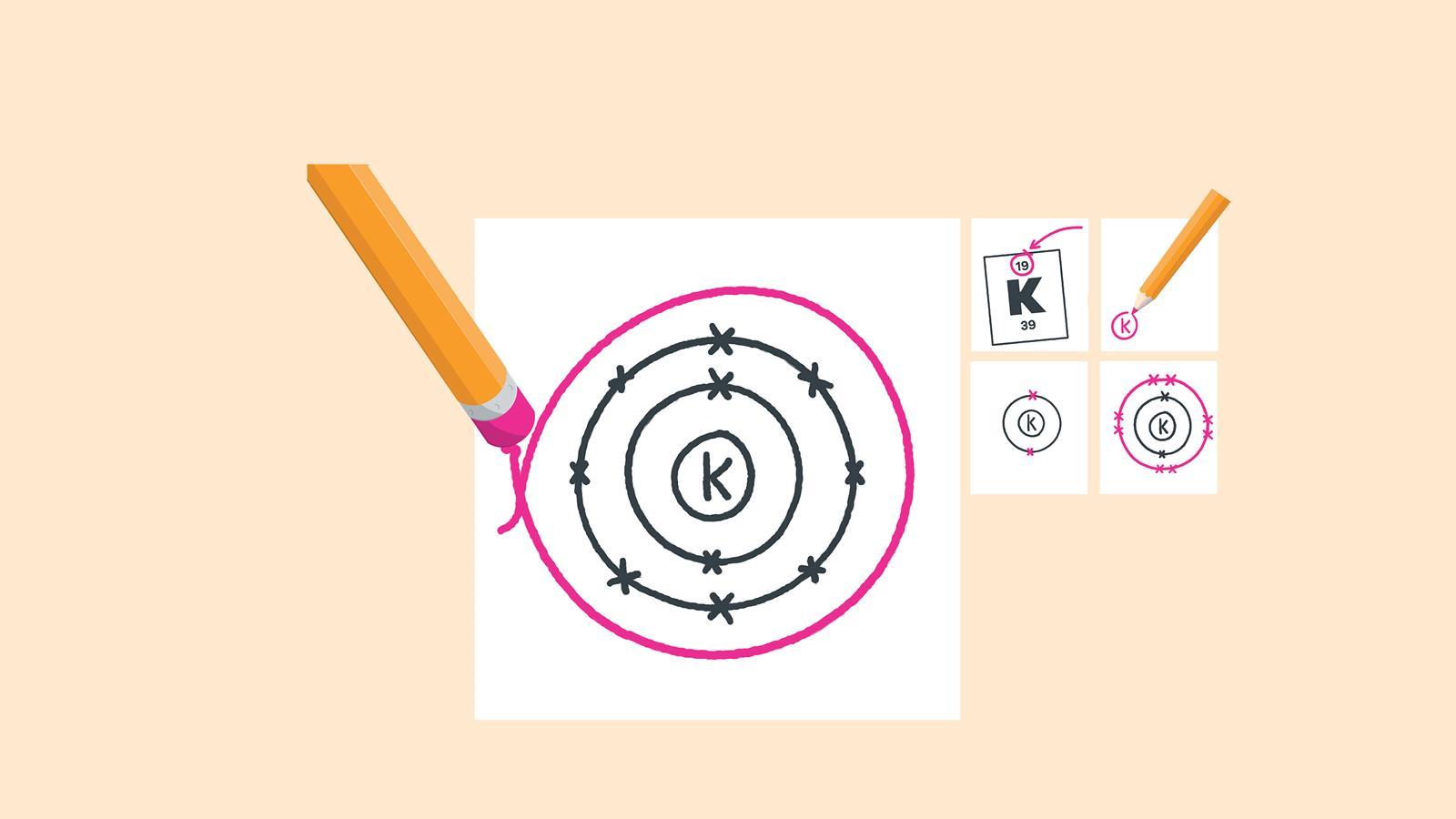
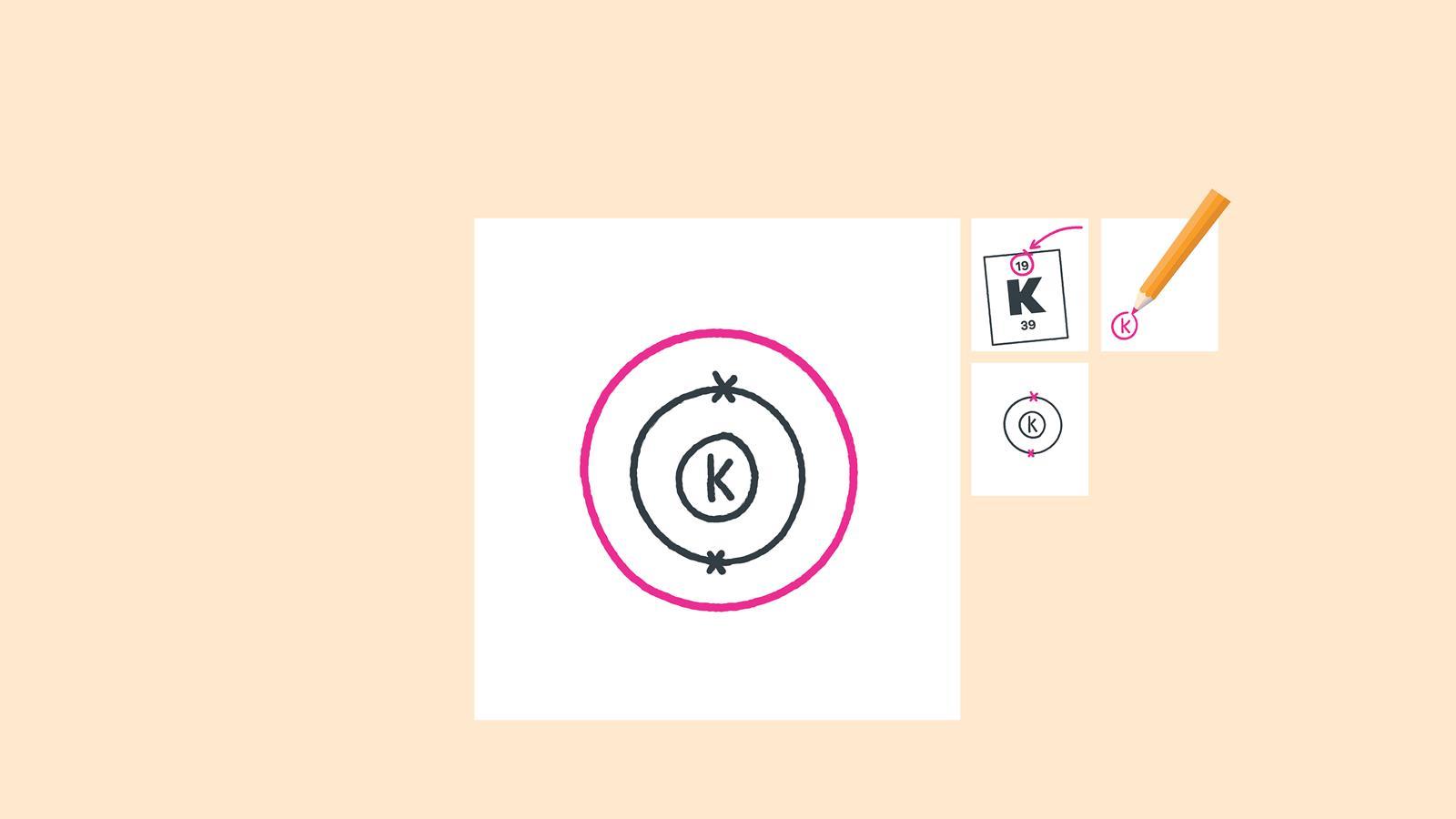
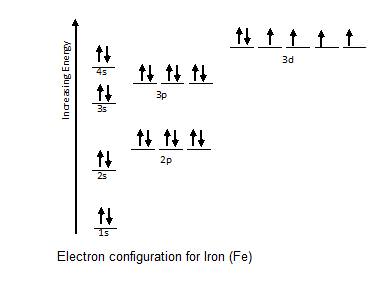
Here, the electron configuration of copper ion (cu +) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10.
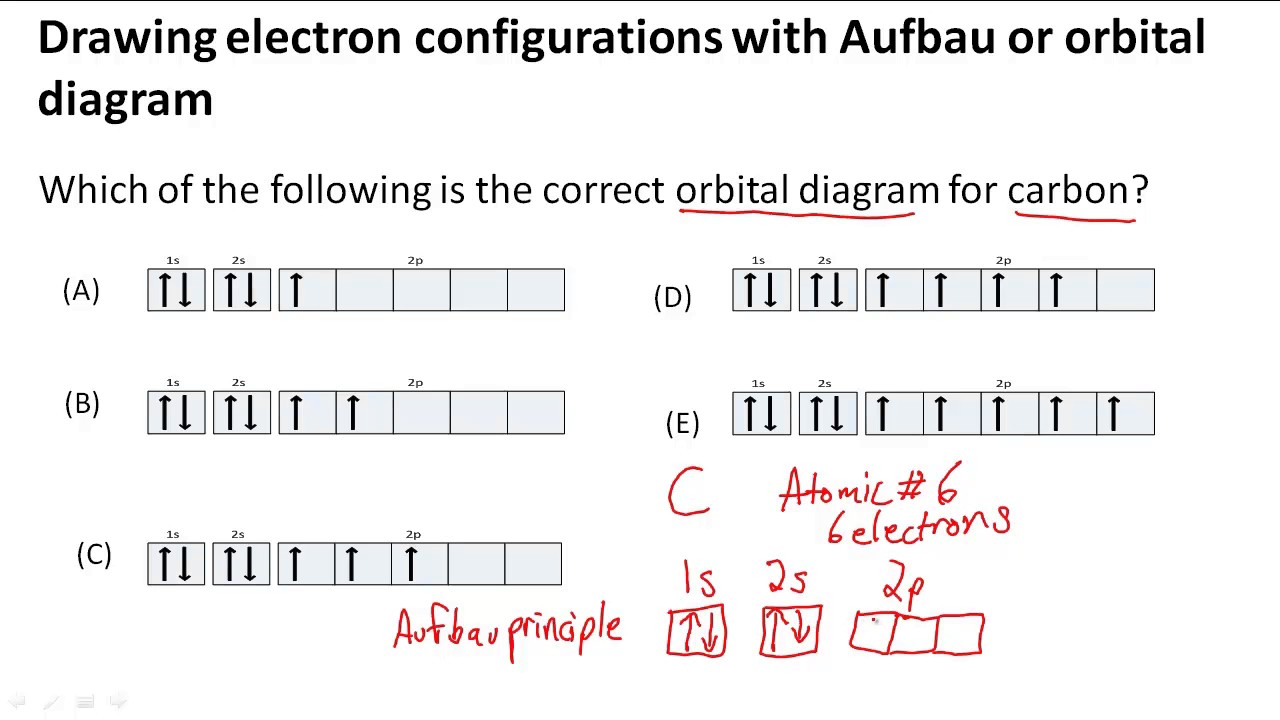
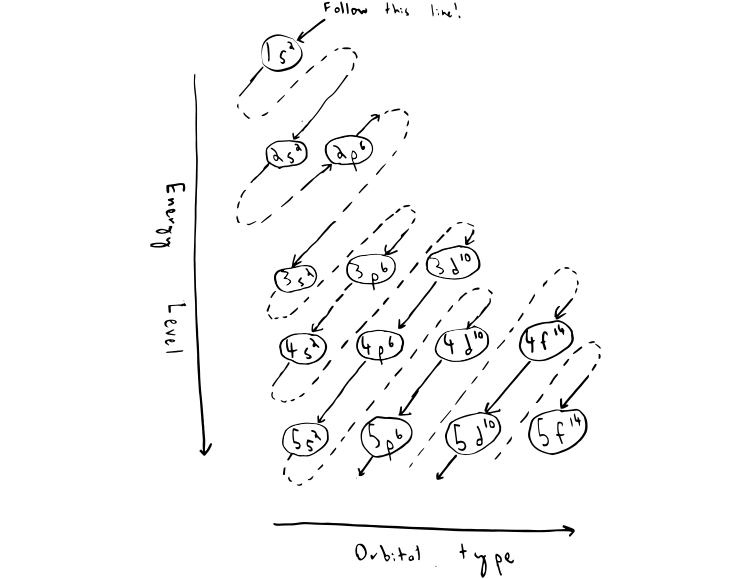
How to draw an electron configuration. Assign quantum numbers to the electrons in. Higher the value of n+l for the orbital, higher is the energy. This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration.
1s 2 2s2 2p6 3s2 ↑↑↑. On the other hand, the copper atom donates an electron in the 4s orbital and an electron in the 3d. If two orbitals have the same value for n+l, the orbital with lower value of n will have the lower energy and so the electrons will occupy.
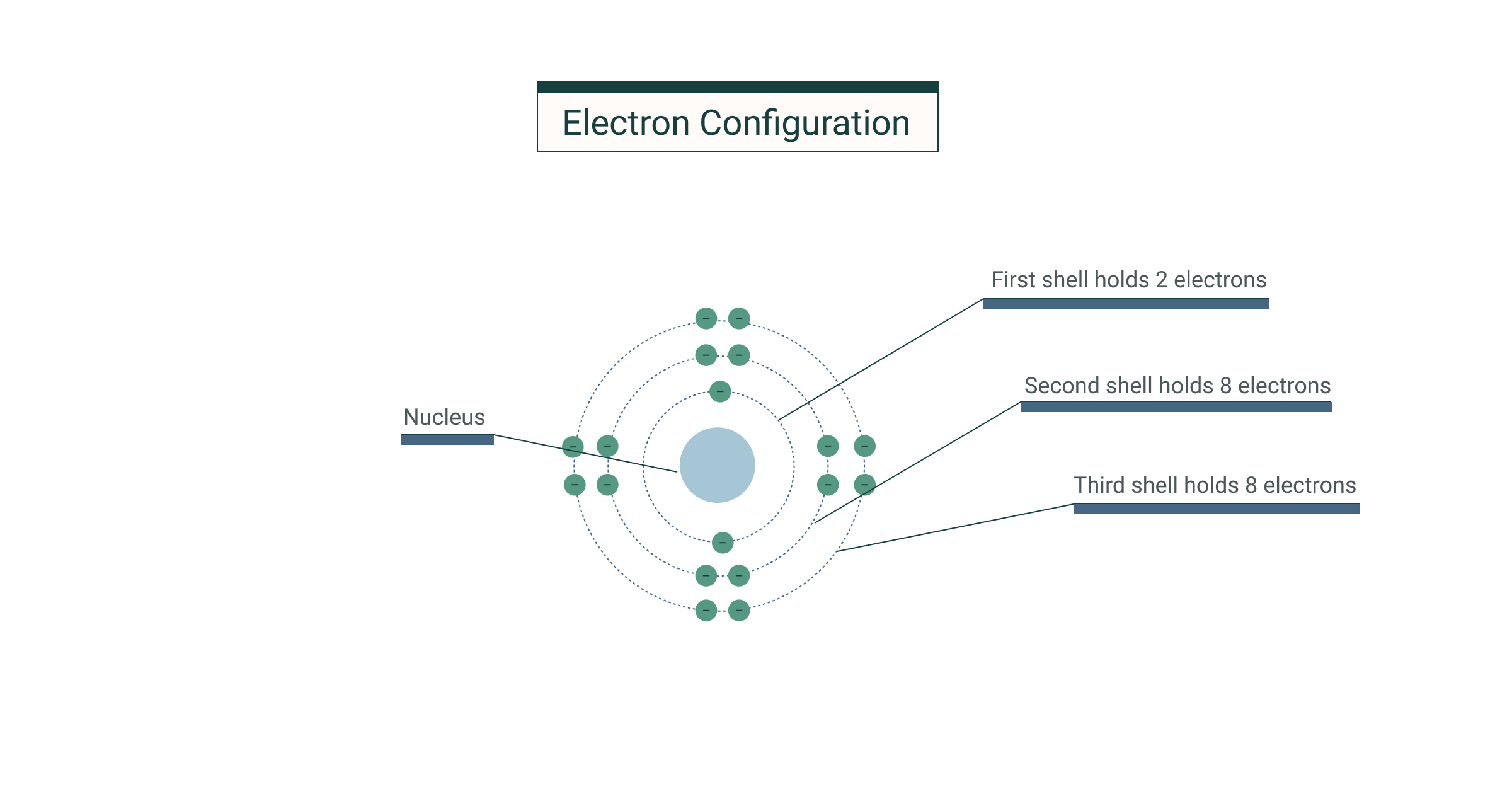
An electron configuration diagram is a model that depicts the. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Electron configurations and electron orbital diagrams electron configurations ex.
It contains plenty of practice problems and examples including the. Layer (row #), s = orbital type , power of 2 = the 2. Write electron configuration for an excited state exercises exercise 1.
The valency of the element is determined by. Therefore, the electron configuration of bromine(br*) in an excited state will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p x 2 4p y 1 4p z 1 4d xy 1.